Rationale for PROMPPT
In light of the lack of evidence supporting the effectiveness of opioids and concerns about the potential harms, best practice guidelines recommend that opioids should be reviewed within 4 weeks of starting opioid treatment, at least 6 monthly once a stable dose is reached, and more often if there are concerns. Structured review is recommended, taking into account evidence of effectiveness, including functional improvement / progress towards treatment goals, side-effects and evidence of problematic use . Supporting patients to taper and stop opioids is recommended if treatment goals are not met.
In practice, implementation of these opioid guideline recommendations is low. Lack of time is an important barrier and the available time in routine GP appointments offers limited opportunity to undertake a comprehensive review.

The expansion in the clinical pharmacist workforce in UK primary care. presents an opportunity to address this. One emerging role for clinical practice pharmacists is reviewing patients with polypharmacy and complex medicines regimens. Given that increasing polypharmacy is associated with incremental increases in long-term and stronger opioid prescribing, practice pharmacists seem ideally placed to take a proactive role in reviewing and managing patients on long-term opioids. Currently, however, there is no evidence about how they should do this or whether it would be clinically or cost-effective. The PROMPPT programme aims to fill this evidence gap.
Aim of the PROMPPT Programme
The PROMPPT research programme aims to develop and test a clinical pharmacist-led primary care intervention (PROMPPT) to reduce opioid use for people with persistent pain (where appropriate) and support self-management for those with persistent pain in primary care.
Research plan
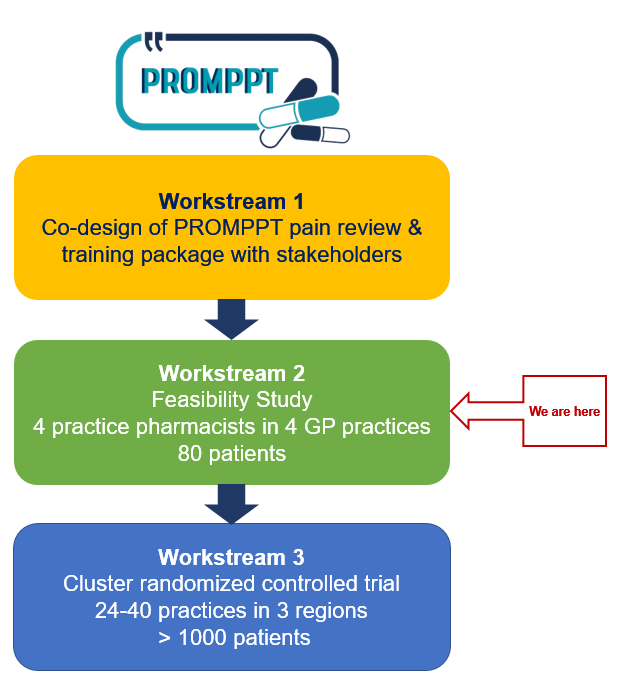
In workstream 1 we combined findings from 4 qualitative studies with best practice guidance and theory to co-design the PROMPPT pain management review and clinical pharmacist training package with a range of stakeholders including patients, clinical pharmacists, GPs and healthcare professionals with pain management expertise.
We are now in workstream 2, a non-randomised feasibility study which will inform refinement of the PROMPPT pain management review and training package as well as the design of the proposed main trial design.
4 practice pharmacists in 4 different GP practices (2 East Midlands, 2 West Midlands region) will take part in the feasibility study, which aims to recruit 80 patients across the 4 practices.
The overall aim of this study is to investigate the credibility and acceptability (to patients, clinical pharmacists and GPs) of PROMPPT, the practicality of clinical pharmacists delivering PROMPPT in primary care and the effectiveness of the training package in enabling clinical pharmacists to deliver the PROMPPT intervention to patients prescribed long-term opioids for persistent pain in primary care. We will also explore the feasibility of the proposed design for a main trial to test clinical and cost-effectiveness.
In workstream 3 a large multicentre cluster randomised controlled trial (cluster RCT) will test the clinical and cost-effectiveness of providing the PROMPPT pain management review. This trial will investigate whether, compared with usual care, providing a PROMPPT pain management review for patients prescribed long-term opioids for persistent pain reduces opioid use (by at least 25% on average), without making pain/pain-related interference worse. We will use mean daily morphine equivalent dose (MED) to measure opioid use.
Which patients are eligible for PROMPPT?

Adult patients aged ≥18 years are potentially eligible if they have been prescribed any opioid-containing analgesic for persistent non-cancer pain continuously for ≥6 months, and a prescription has been issued within the previous 2 months.
Exclusions
Patients with acute pain, cancer pain and/or terminal illness (life expectancy less than 6months);
Vulnerable patients (e.g. severe mental illness, learning difficulties, dementia);
Patients currently receiving treatment for substance misuse;
Patients who are unable to understand English.
Objectives of the PROMPPT pain management review
PROMPPT aims to reduce opioid use for people with persistent pain where appropriate.
It may not be appropriate for all patients to reduce opioids and some patients may not yet be ready to reduce their opioids. So it is important to recognise all the potentially useful outcomes from an individual PROMPPT pain management review.
Examples of ‘successful’ outcomes of PROMPPT pain reviews :
● The patient successfully reduces or stops opioids – this is the ‘ideal outcome’ where opioid reduction is appropriate.
● Opioids stay the same because benefits outweigh harms/risks (e.g. low dose / intermittent with evident functional benefit) AND the patient is now aware of the potential risks of opioids, the role of self-care in managing persistent pain and understands why it’s important to review opioid use. There may also be scope to reduce ineffective / potentially harmful non-opioid pain medicines.
● Opioids stay the same because the patient does not feel ready to try reducing opioids BUT is now aware of the potential risks of opioids, the role of self-care in managing persistent pain and understands why it’s important to review opioid use. This may ‘sow a seed’, leading to the patient reflecting on their opioid use / pain management strategies and they may consider making a change in future. There may also be scope to reduce ineffective / potentially harmful non-opioid pain medicines.
● Opioids stay the same even though the patient wanted to escalate, potentially harmful escalation has been avoided AND the patient is aware of the potential risks of opioids, the role of self-care in managing persistent pain and may be less likely to escalate or request escalation of their opioids in future. As above, this may ‘sow a seed’, leading to the patient reflecting on their opioid use / pain management strategies and they may consider making a change in future. There may also be scope to reduce ineffective / potentially harmful non-opioid pain medicines.

