This lesson introduces some of the aspects of the PROMPPT review that are specific to the research study, including the documentation that you will be asked to complete as part of the research process. For a more detailed overview please refer to training manual section 1.5.
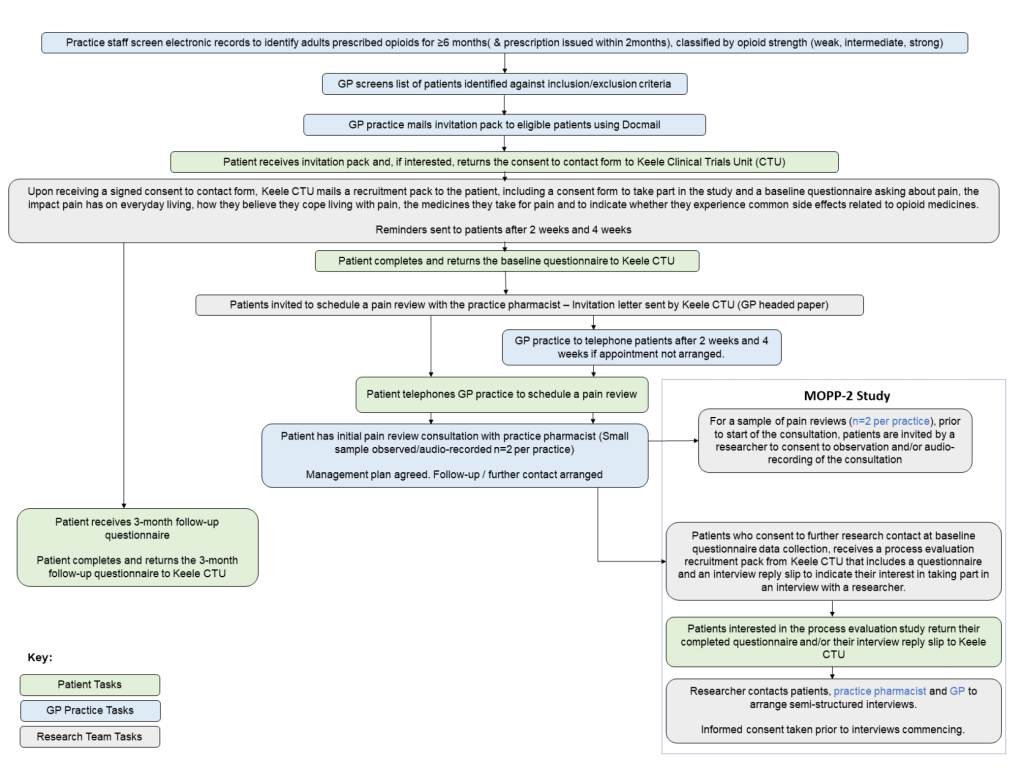
The PROMMPT feasibility study primarily consists of three components:
- Questionnaire study- patients will complete a baseline questionnaire and a follow-up questionnaire at 3 months. The patient facing name of this part of the study is MOPP (Management of Opioids & Persistent Pain).
- PROMMPT pain management review- patients will be invited to book a consultation with the clinical pharmacist at the practice.
- Process evaluation- a sample of consultations will be observed and/or audio-recorded. Patients will be invited to take part in a semi-structured interview with a researcher, the clinical pharmacist and GP lead for the study at the practice will also be interviewed. The patient facing name of this part of the study is MOPP-2.
Involving the whole practice
The involvement of the whole practice (including administrative and healthcare professional team members) is important to enable the delivery of the feasibility study and to support the provision of joined-up care for individual patients.
Practice staff may be the first point of contact for the patients regarding taking part in the PROMPPT feasibility study. All documentation for the questionnaire study and the process evaluation signposts the patients to the research study team at Keele University. However, we anticipate that patients may contact the practice with questions regarding the research study. With this in mind:
- Administrative teams will be provided with some FAQs that may help to address some questions that the patients may ask.
- If a question is asked that the practice staff member is unsure of the answer, we will encourage them to pass on the research team’s contact details to the patient.
During the PROMPPT pain management review process (first assessment and subsequent follow-ups), patients may consult with another member of the healthcare team. For this reason, we encourage all clinical practice staff:
- To be aware of the PROMPPT feasibility study
- To be familiar with the aims of PROMPPT
- To be consistent in the communication about the aims of PROMPPT with patients
To support this approach, the training manual will be made available for other staff members, including a study summary that easily explains the PROMPPT feasibility study and what taking part in the study as a practice will involve. Consultation records based either in EMIS or SystmOne will identify when a patient has attended a PROMPPT review.
Study documentation
We have developed three forms for completion as part of the PROMPPT intervention, two case report forms (CRF) and one patient-facing form called the “pain review plan & self-care information”.
The purpose of completing the CRF is to provide a clinical and research record of the consultation. The document is easy to complete and takes you through each stage of the consultation step-by-step. As you would in a normal consultation, it is important that the documents are completed within or shortly after the consultation.
We then ask you to email a copy of the completed CRF for that PROMPPT pain management review. We would like you to email CRFs for all PROMPPT pain management reviews that take place (or are scheduled to take place) up to 3-months from the first scheduled PROMPPT pain management review. Within this 3-month time-frame, please email all CRFs to our PROMPPT study email address, sch-tr.studypromppt@nhs.net, on the day of the consultation. For PROMPPT consultations that happen beyond this point, please continue to use the CRF for the clinical record only.
The pain review plan & self-care information was developed to be a patient facing document and should be completed by you alongside the patient. We do not need you to email us a copy of this record.
Later in the training we will look at how to find each of the study documents in the consultation and how to complete them. A copy of these documents can be found in the PROMPPT resource library on this platform.
Safety reporting
Patient safety is of paramount importance in clinical research and safety reporting is a critical part of the PROMPPT feasibility study. The PROMPPT pain management review is considered to be evidence-based best practice and has the endorsement and credibility of the clinical community. Given this, the potential harms of this feasibility study are considered to be minimal. However, we can only confirm this via the safety reporting and review process.
What is an adverse event?
An adverse event is any medical occurrence that may or may be related to, or a consequence of participation in a research study. A Serious Adverse Event (SAE) in the context of this study would be defined as an untoward occurrence that:
(a) results in death;
(b) is life-threatening;
(c) requires hospitalisation;
(d) results in persistent or significant disability or incapacity;
(e) is otherwise considered medically significant.
We know that reducing opioids can result in a transient increase in pain and/or withdrawal symptoms but these events are not considered to be SAEs.
Reporting an adverse event
It is important to report an SAE as soon as you, or the patient’s GP become aware of them during the study. The flow chart below outlines the procedure that should be taken.
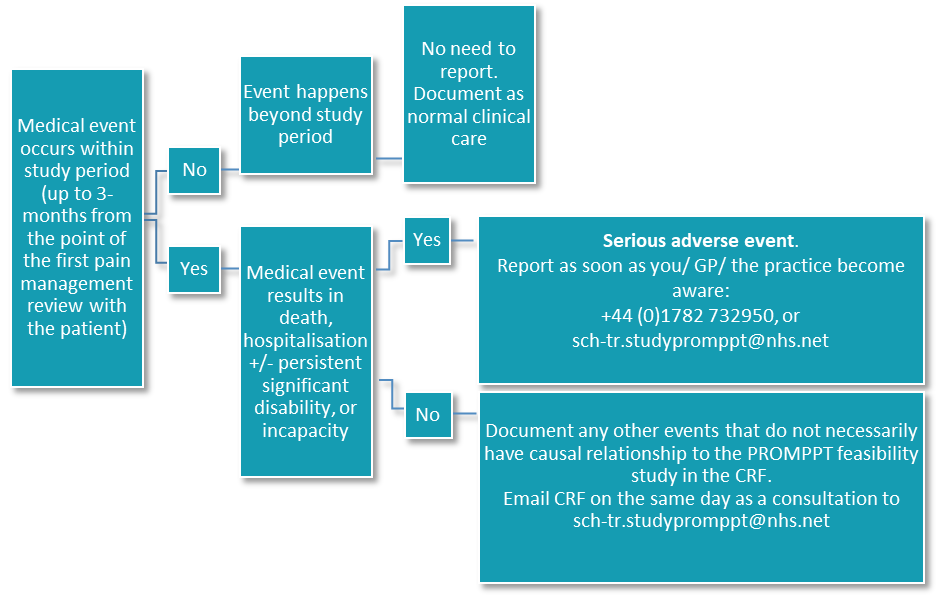
Adverse events that are not SAEs do not need to be reported, however, it is important to record adverse events for your clinical record as well as for research purposes. You should record any adverse event on a CRF and email the research team on the same day as the appointment with a patient.
Take me to: Foundation Module Quiz

